
The oxygen has a formal charge of 6 - 4 - 2 = 0 (same ordering of terms). The central carbon has a formal charge of 4 (valence electrons) - 0 (lone pairs) - 4 (bonds) = 0. Hence, The formal charge and P-O bond order in PO43- respectively are -0.75 and 1.25. Another way of saying this is that formal charge results when we take the number of valence electrons of a neutral atom. All atoms in the molecule have zero formal charge, the "happiest" situation for any molecule. It is the difference between the valence electron number of the atoms and the combined sum of bonded and non-bonded electrons in an atom. The formal charge of an atom in a molecule is the hypothetical charge the atom would have if we could redistribute the electrons in the bonds evenly between the atoms.
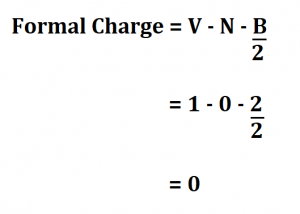
Each hydrogen atom has one electron as a neutral atom, no lone pairs and shares one bond, for a formal charge of zero. It has no lone pairs, and it shares four bonds, so the formal charge is zero. The carbon in CH 4 has four electrons as a neutral atom. Subtract the number of bonds shared by the atom.Subtract the number of non-bonding electrons (usually in lone pairs).The balancing chemical equations calculator balance the charge by adding. Count the number of valence electrons of the neutral atom. In this example, the nitrogen and each hydrogen have a formal charge of zero.

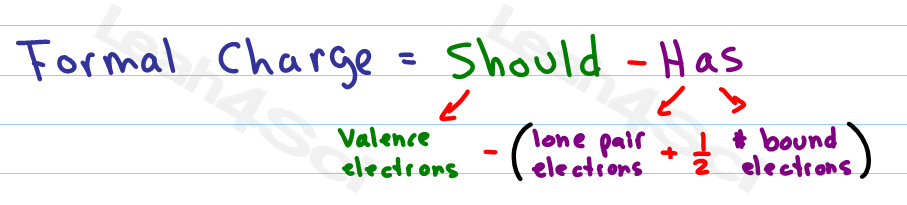
The sum of the formal charges, with a couple of extra rules, will help us to decide which of multiple-possible valid Lewis structures is likely to be the correct one. This suggests that option A is the correct answer. Formal charge is just a way of bookkeeping that helps us to decide which of multiple Lewis structures is the likely true bonding arrangement of a covalent molecule. The formal charges on the nitrogen atoms in the case of azide ion from left to right are -1, +1, -1. In those cases we resort to calculating what's called the formal charge of each atom. a Closer looK In Section 3-8 you learned to calculate the oxidation number of an atom as a. Sometimes it's difficult to tell which of two possible Lewis structures of a compound represents the actual bonding of the molecule.


 0 kommentar(er)
0 kommentar(er)
